The new clinical practice guideline was developed by the ADA and funded by an FDA-provided grant.
Oral Health
News and Features
Current evidence is inadequate for assessing balance of benefits and harms of screening in asymptomatic children.
The US Food and Drug Administration (FDA) approved a wide range of therapies in 2023, including many new molecular entities and biological products. The information below is organized by the month in which the product received approval.
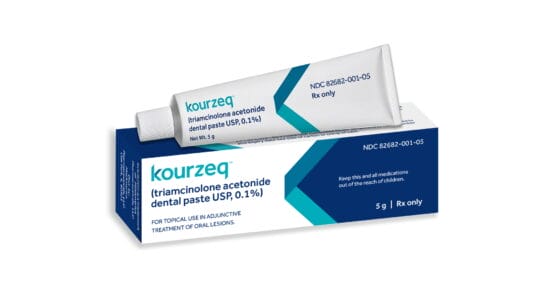
Kourzeq is supplied in tubes containing 5g of dental paste, and is available exclusively via telehealth through a partnership with BeyondMD.
The ADA guideline aligns with FDA recommendations advising against codeine and tramadol use in pediatric patients.
Two draft recommendation statements find lack of evidence for dental screening, preventive interventions in children, adults.
Use of fixed palatal expansion devices in adults has been reported to lead to serious complications.
No significant difference observed in terms of tongue coating scores, plaque index for those receiving probiotics
More adverse dental outcomes seen with sublingual buprenorphine/naloxone compared with use of transdermal buprenorphine or oral naltrexone
No increased perioperative morbidity seen among patients with local regionally advanced oral cavity squamous cell carcinoma
No evidence of temporal association seen between invasive dental procedure and late prosthetic joint infection
This week news of a new treatment for insomnia; The FDA comment on magnesium hypertension claims; Transmucosal buprenorphine linked to dental decay; Researchers assess changes in menstrual cycles in relation to COVID vaccines; And experts have their say on the best diets for 2022.
Primary care clinicians should apply fluoride varnish to the teeth of all children younger than 5 years of age to prevent dental caries
Additional benefits include reducing variability and significant cost savings
Fluoride supplementation recommended for children 6 months and older if water supply does not contain enough fluoride
The recall applies to products bearing an expiration date from 11/2021 through 5/2022.
Paroex Chlorhexidine Gluconate Oral Rinse is indicated for the treatment of gingivitis.
Psychiatric, orthopedic, and sleep issues most commonly cited in a Reddit forum
In older adults, interactions with meds for depression, anxiety increase risk for ED visits, hospitalizations.
“The data indicate that more conservative periodontal therapy might initially be considered for IBD patients with advanced periodontal disease for whom PPI are prescribed as a component of their IBD treatment,” the authors concluded.