Improvement seen in all signs of photoaging as early as week 4 and through 12 weeks, with no major irritation
Ophthalmic Disorders
News and Features
Both trials met the primary and secondary near vision improvement endpoints.
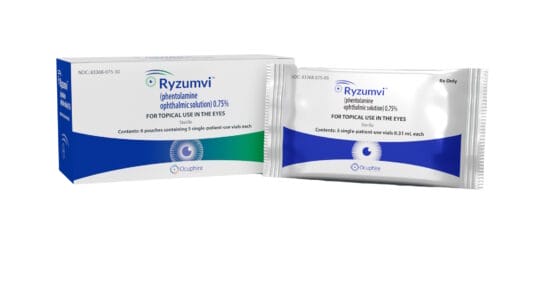
Ryzumvi is a preservative-free eye drop formulation of phentolamine mesylate, a nonselective alpha-1 and alpha-2 adrenergic antagonist that reduces pupil size.
GA enlargement rate in study eyes did not differ significantly in run-in and treatment phases in age-related macular degeneration.
The approval was based on data from two phase 3 trials, which evaluated Clobetasol Propionate Ophthalmic Suspension 0.05% in patients with at least 10 cells in the anterior chamber after cataract surgery.
FDA withdraw multiple myeloma treatment after a confirmatory trial showed an increased risk of death; latest efficacy results for Abrysvo vaccine; chikungunya virus vaccine recommendations; Complete Response Letter issued to investigative schizophrenia treatment; new treatment for complicated UTIs including pyelonephritis; over-the-counter eye ointments recalled.
Trainees and specialists rated chatbot’s accuracy and completeness more favorably than those of their specialist counterparts.
The recalled products include Equate Lubricant Eye Ointment, Equate Style Lubricant Eye Ointment, CVS Health Lubricant Eye Ointment.
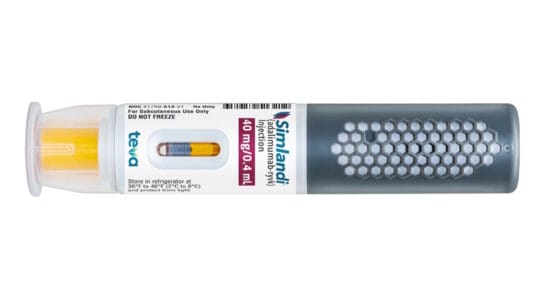
Simlandi is an interchangeable biosimilar and can be substituted for the reference product without requiring a prescription change.
Biogen discontinue Alzheimer treatment; The American Academy of Dermatology issue new acne vulgaris management guidelines; Update for ongoing Paxlovid availability; Narcolepsy/ADHD treatment recalled; FDA warn against copycat eye drop products.
South Moon, Rebright, or FivFivGo eye drops are unapproved drugs with claims to treat eye conditions such as glaucoma.
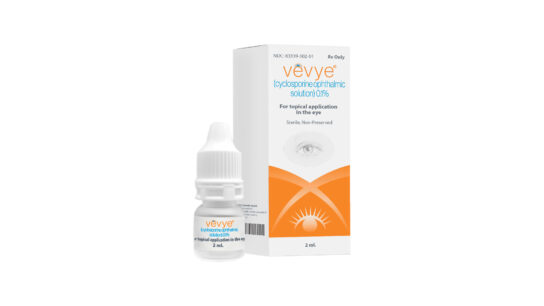
Vevye is supplied in a 5mL multiple-dose eye drop bottle containing 2mL of solution.
The US Food and Drug Administration (FDA) approved a wide range of therapies in 2023, including many new molecular entities and biological products. The information below is organized by the month in which the product received approval.
The Company received 180-day competitive generic therapy exclusivity from the FDA.
Sodium-glucose co-transporter 2 inhibitor use was associated with significantly lower risks for diabetic retinopathy compared with the use of dipeptidyl peptidase-4 inhibitors, sulfonylureas, and pioglitazone.
The PDUFA date refers to the deadline set by the US Food and Drug Administration for reviewing drug applications.
The table below is a review of notable updates that occurred in November 2023 for investigational products in development (not an inclusive list). Click on the status to view our full coverage.
iDose TR is a sterile intracameral implant designed to continuously deliver travoprost for extended periods of time.