The investigational product is an extended-release tablet formulation of diazoxide chloride, the crystalline salt of diazoxide.
Metabolic Disorders
News and Features
A phase 3, open-label study (MEND-PNPO) evaluating MC-1 in patients with confirmed PNPO deficiency (via genetic analysis) is currently in the works.
Sapropterin Dihydrochloride Powder for Oral Solution is indicated to reduce blood phenylalanine levels in patients with hyperphenylalaninemia due to tetrahydrobiopterin-responsive phenylketonuria.
Prademagene zamikeracel is an autologous cell therapy that consists of epidermal sheets that deliver functional COL7A1 genes into the patient’s own skin cells to enable normal type VII collagen expression and facilitate wound healing.
Findings seen in trial where calories were held constant for both groups.
The shortage is expected to continue at least through the second quarter of 2024.
The double-blind, phase 3 study included 2 groups of patients, those who were not on positive airway pressure therapy and those who were.
LX2006 (AAVrh.10hFXN) is an adeno-associated virus gene therapy candidate designed to deliver a functional frataxin gene to cardiac cells to improve mitochondrial function.
Metabolic agents were the most costly category; antidiabetic agents were the mostly costly therapeutic area.
Greater weight loss observed over five years with ESG versus semaglutide for individuals with class II obesity.
The table below is a review of notable updates that occurred in March 2024 for investigational products in development.
No increase in aspiration or subsequent pneumonia seen among patients undergoing surgical procedures
Larger reductions seen in heart failure-related symptoms and physical limitations for patients with HFpEF, type 2 diabetes
Elamipretide is a peptide compound designed to penetrate cell membranes targeting the inner mitochondrial membrane where it binds reversibly to cardiolipin.
Benefits seen across anthropometric, metabolic, biomarker, and psychiatric outcomes
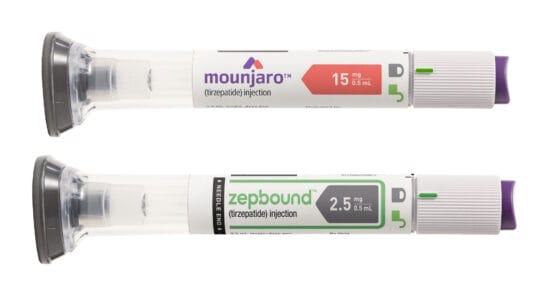
Zepbound is indicated for use as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management.
As polygenic risk score increases for BMI, number of daily steps associated with lower risk for obesity increases.
The application is supported by data from the pivotal phase 3 IB1001-301 trial, which evaluated the efficacy and safety of IB1001 in patients 4 years of age and older with NPC.
The approval was based on data from the randomized, double-blind, placebo-controlled phase 3 EPIDYS study.